Data4GO – Your intelligent documentation software
GermanOncology has developed Data4GO, a modular and completely web-based documentation software for the collection and evaluation of medical study data.
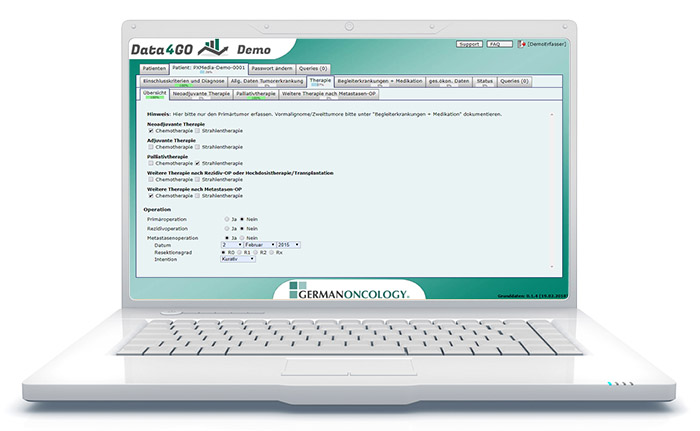
The functionality of the Data4GO software
Data4GO serves the collection and evaluation of medical study data. The advantages of a Web application are combined with the interaction possibilities of a desktop application. The result is a modular platform with which individually tailored documentation interfaces for studies can be quickly created.
Participating physicians call up the studies in a user-friendly manner using a link and individualised access data via their web browser – without installing additional software. Unlike many other online systems whose predefined workflow does not allow any interruptions in the collection of data, Data4GO users are free to collect the data in a sequence they like and to supplement or modify it at any time until the end of the study. All changes are documented in history and can be tracked for quality assurance. Inadmissible information is displayed immediately. Data4GO behaves like a desktop application with immediate feedback.

The most important Data4GO features at a glance:
Collection and evaluation of oncological, medical study data
Fully anonymised patient records
Data can be entered anytime and anywhere, including retrospective documentation
Adding individual functions, quick additions or changes to data fields through modular design and central control
Graphical evaluation of important characteristics in real time - enables, among other things, better control of recruitment
Service from a single source: fast and effective assistance and problem-solving
No additional software installation necessary, as access is via a secure SSL connection (https)

More Data4GO functions:
Predefined selection lists to standardise data designations and shorten documentation times
Acquisition according to individual sequence and time
Checks the entered data against predefined value ranges
Completeness check for mandatory fields
Immediate display of incorrect or inadmissible data
Corrections of the documented values can be made at any time
Data records can be locked to prevent changes after the evaluation has been completed
If required, documentation can be carried out centrally by GermanOncology
Revenue- and Cost Calculators
GermanOncology has developed web-based revenue and cost calculators based on reimbursement processes and fee systems for various tumour diseases.

Revenues and costs under various reimbursement conditions
We offer you license models for therapies in oncological, urological, haematological and psychiatric areas, among others, but we also offer new calculators, individually designed for your needs. Therapies under inpatient, day-care and outpatient conditions are modelled and represented graphically, taking regional conditions into account. You are free to choose the length of your access: The different license models are available for 12, 24 or 36 months.
Advantages of a web-based revenue calculator:
The licenses include the online and offline use of the calculators, without additional installations
Flexible use from any Internet-enabled computer, tablet and smartphone (including iOS)
Optional consideration of personnel and material costs/infrastructure
Input option for individualised parameters (e.g. daily rates, quarterly flat rates and unweighted additional charges)
Options for regional parameters (e.g. 17 “KV” districts, country base case values, oncology agreements)
Revenue displays on, e.g. cycle, quarterly and average patient basis
Display by positive/negative bar charts with consideration of costs for personnel and infrastructure for quick overviews
Our calculators provide information about:
Proceeds from medical services under various forms of outpatient care (including statutory health insurance physician, § 116b, authorised hospital doctors, a day clinic, university outpatient clinic).
Proceeds from drug supply for all current treatment regimens for one indication
Revenue from medical services and medication supply
Revenue from inpatient care with/without additional charges
Revenue from contractual medical services in 17 “KV” districts
Revenues from various chemotherapy applications (e.g. intravenous versus subcutaneous)
Comparison of revenues from different therapies under different care structures and specialist groups within one indication




